The National Medical Products Administration (NMPA) of China released data on medical device approvals in Jul 2023. A total of 197 products were approved by the NMPA for market release, of which, 150 were domestic and 47 were import products.
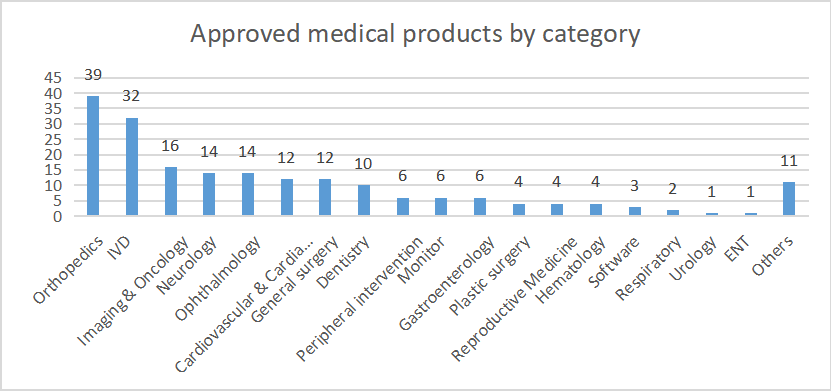
As a comparison, in Jul 2022, 197 medical device products were approved by the NMPA. To date, a total of 1,392 approvals have been made by NMPA this year, a rise of 2.9% from the 1,353 approvals in Jan-July 2022.
